A sterilized carabid beetle before gut dissection.
Angela Gadino, WSU
Do you ever wonder what those earwigs, spiders, and other ground-dwelling predators eat in your orchard?
This question has been a main focus in the Enhancing Biological Control in Western Orchards Specialty Crop Research Initiative project. We know these predators are in our orchards, but up until now who is eating what has not been well documented, especially with regards to codling moth.
We set out to look at which major predator groups found commonly in western orchards might be eating the most codling moth larvae.
Codling moth larvae, once fully grown inside apples or pears, leave the fruit during summer and fall and seek cocooning sites from which they either emerge as second-generation adults or overwinter as mature larvae until the following spring. The cocooned larvae can be found in bark crevices on trees, on props, or in leaf litter and other organic matter on the orchard floor.
Generalist predators active in the tree canopy, the trunk, or on the ground may discover and feed on codling moth larvae in these places. We believe that predation on these codling moth larvae, either moving to or in cocooning sites, can help reduce population levels in the following season. However, we needed real numbers to support our beliefs.
But what is the best way to determine which of the predators we find in orchards are eating codling moth larvae in the field? Many of these lower canopy and ground-dwelling predators are cryptic in their feeding behaviors. Most predators are only actively hunting for prey during the night, and they like to hide in cracks, crevices, ground cover, leaf litter, or soil. Also, unlike parasitism, it is difficult to find evidence of predation since usually the whole body of the prey is consumed. Fortunately, new molecular technologies allow us to analyze the gut content of predators, which gives us a better picture of who is eating codling moth.
The technique is relatively straightforward, using a primer in a PCR (polymerase chain reaction) analysis that amplifies any codling moth DNA that occurs in the gut content of each predator tested. The tricky part is capturing the predators in the orchard and collecting them before they have the chance to fully digest their prey so there is still DNA material available to analyze.
Pitfall traps
To do this, we used pitfall traps, which are plastic cups sunk into the soil, where the lip of the cup is level with the ground so unsuspecting insects will fall into the traps. Since most of these predators are active at night, the insects or spiders captured in the cups were collected each morning. The samples were then brought to the laboratory where they were sorted into groups and frozen until ready for analysis.
The molecular analysis consists of two main parts. First, the frozen specimens were thawed, sterilized, and placed into small plastic vials and homogenized with a buffer solution. For some of the larger species, like ground beetles, the guts were dissected and used instead of the whole insect body.
The homogenized samples were then ready to undergo PCR amplification, allowing us to detect codling moth DNA. Polymerase chain reaction analysis consists of several steps where a small sample of the homogenized predator solution is added to a buffer mix containing a special enzyme that copies DNA, as well as short pieces of DNA called primers, to ensure that only codling moth DNA is amplified.
We also add the building blocks needed to create new DNA strands, and a fluorescent dye that allows us to measure the amplified DNA. The mixture is then processed, and amplified DNA assessed by measuring fluorescence.
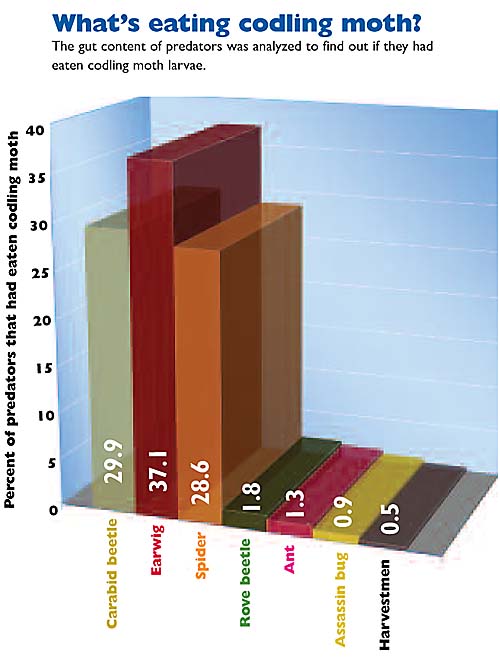
In over 2400 samples collected from seven orchards during a three-year period, we found that about 9% had codling moth DNA in their guts. The majority of the samples showing codling moth predation came from three main groups: carabid ground beetles (Carabidae), the European earwig, and spiders (Araneae).
We also found that predation rates varied between the different orchards sampled and were linked to management intensity. Pooling across all seven orchards, we found 8.5% of the ground beetles, 8% of the spiders, and 16% of the earwigs had fed on codling moth.
Many prey types
These results revealed which predators are likely to be important as biological controls for codling moth, and challenge us to create pest management programs focused on conserving them while continuing to adequately control codling moth and other important pests.
We should also remember that these predators are generalists and feed on many prey types, including each other. Feeding on codling moth is just one indicator of their importance, as they are also likely preying on other pests such as leafrollers and wooly apple aphids. Predators, such as ground beetles, may also be particularly important in the newer high-density orchards where codling moth is more likely to overwinter on the ground, since trees in these orchards tend to have smooth bark and thus provide fewer cracks and crevices for the larvae to cocoon.
It is clear that biological control alone will not control codling moth to acceptable levels, but these predators can contribute to reducing codling moth population levels. In turn, lower pest pressure will allow mating disruption, softer materials, and possibly fewer cover sprays, to provide more effective controls. Understanding the ecological role of these predator groups will help shape future orchard management programs while enhancing biological control.
This is the fourth article in an eight-part series highlighting results of a five-year Specialty Crop Research Initiative project to enhance biological control of orchard pests. The project involves researchers from Washington State University, Oregon State University, University of California Berkeley, and the U.S. Department of Agriculture in Yakima, Washington.
Leave A Comment