
2020 saw significant mildew pressure in numerous regions and orchards of Washington, especially those planted to susceptible cultivars, such as Honeycrisp and Granny Smith, which now occupy a large portion of the state acreage. The significant increase in organic acreage may also explain some recent patterns. A four-year study undertaken by Washington State University researchers sheds light on the disease cycle, spread, virulence and sensitivity to fungicides, as well as providing some management clues.
Apple mildew has a mixed reproduction system and spreads frequently between neighboring orchards.
Apple powdery mildew, caused by the fungus Podosphaera leucotricha, is common in apple orchards of Central Washington and several other growing regions. We recently assembled the first full DNA sequence of P. leucotricha — a resource that will certainly open up more research opportunities that were not previously possible because it is impossible to grow this fungus in the lab on artificial media, like many other fungal pathogens. New DNA markers have been developed from this full sequence and have been used to analyze how P. leucotricha produces inoculum and how diverse its population is in U.S. apple orchards from Washington, New York and Virginia.
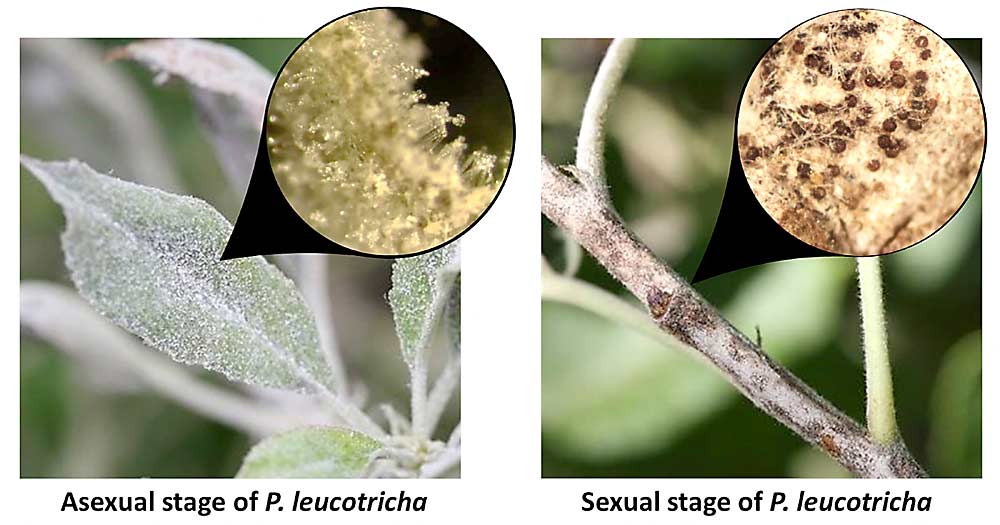
In contrast with cherry mildew, it has been thought for a long time that P. leucotricha reproduces only clonally by overwintering as dormant mycelia in buds and then producing asexual spores in spring and summer. However, sexual structures are not rare in Central Washington and can be seen in the form of small black fruiting bodies on the surface of infected shoots. We discovered that isolates of P. leucotricha can be differentiated by two opposite sexual mating types, like male and female sexes in humans, and both mating types were found in Washington orchards. This sexual reproduction produces the black fruiting bodies. These structures seem to form on young shoots and carry over to the following spring, when we suspect they play a secondary role in initiating new infections. Therefore, a good winter pruning of shoots carrying such structures will reduce inoculum size at the start of new seasons.
Although additional studies are needed on apple mildew populations from Eastern U.S. regions, our results indicate that mildew populations from Washington are genetically different from those of New York and Virginia. This means that the mildew populations emerge and adapt to local environments. Even within Washington, P. leucotricha populations collected in 2018 and 2019 from two organic and six conventional Granny Smith orchards (stretching between Okanogan and Pasco) showed high genetic diversity. The presence of sexual structures and two sexual stages of the fungus in the regions drives that diversity, which could be a sign that the fungus may be more challenging to manage if new mutants or variants arise in the future.
More importantly, we found that apple mildew inoculum is frequently wind-spread between neighboring orchards that are 10 miles apart in Central Washington, and, while rare, spread may occur between orchards that are as far as 60 miles apart. Such spread was not detected between Eastern U.S. apple orchards. Given the intensive production system along the Columbia River in Central Washington, inoculum spread between orchards may be more frequent than we previously thought. This finding may reflect a “neighbor effect” that should be considered in mildew management programs to optimize spray types and timing. Our findings also highlight the critical role of nurseries in producing disease-free trees and the importance of plant material transportation between states to limit spread of potential new fungal genotypes or variants.
No evidence found for new virulent variants or emergence of fungicide resistance in P. leucotricha populations in U.S apple orchards.
A piece of good news is that we did not find new variants of the apple mildew pathogen P. leucotricha emerging with the ability to break the resistance conferred by a major resistance gene in apple mildew-resistant cultivars. Our study was mostly done on populations collected from the susceptible cultivars Granny Smith and Honeycrisp, and although studies including other cultivars will be important, we believe that the risk of major resistance breakdown is low. Moreover, the full DNA sequence we obtained will be instrumental for future apple breeding efforts.
We also found no evidence of fungicide resistance development in P. leucotricha to fungicides from major FRAC groups 3, 7 and 11, which are commonly used for mildew control in U.S. apple orchards (see Figure 1).
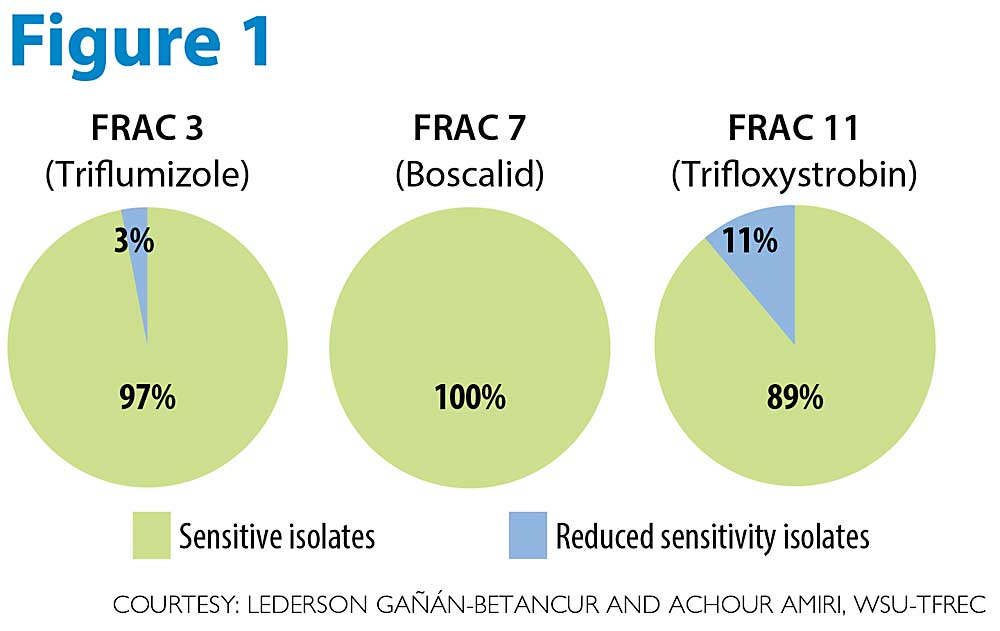
While the apple mildew fungus has a lower risk of developing fungicide resistance compared to the cherry mildew fungus, FRAC 3, 7 and 11 fungicides are considered either medium or high risk for resistance development by the Fungicide Resistance Action Committee (FRAC). We tested more than 250 P. leucotricha isolates from organic and conventional orchards for sensitivity to triflumizole (Procure, FRAC 3), boscalid (Pristine, FRAC 7), and trifloxystrobin (Luna, Flint, FRAC 11). We found that all isolates were fully sensitive to boscalid versus 97 percent for triflumizole and 89 percent for trifloxystrobin. Although a very small portion of isolates showed “reduced sensitivity” to triflumizole (3 percent) and trifloxystrobin (11 percent), these isolates were still fully controlled by the label rates of both fungicides.
In Central Washington, the absence of actual fungicide resistance to tested fungicides is good news and is certainly explained by the relatively lower fungicide input in this region where apple scab is not an issue. However, caution is recommended when using current fungicides, to avoid selecting for resistant isolates from those that have already developed reduced sensitivity. Rotation of fungicides from these FRAC groups with others from FRAC groups 1, 9, 19 and M02, in addition to other organic materials, is the way to go. We are confident that the bioassay and molecular markers developed in our recently published study will ease future fungicide resistance monitoring of this fungus. High apple mildew incidence has been reported by Cornell University in an experimental Jonagold orchard in New York sprayed with the FRAC 3 fungicides myclobutanil (Rally) and flutriafol (Rhyme) in 2019. That suggests some resistance to this group may have developed there, and studies including larger populations from the Eastern regions with higher fungicide input are warranted to shed more light on the big picture of fungicide resistance in the Eastern U.S.
New materials are available for mildew control in organic orchards.
Our recent orchard trials conducted on Granny Smith and Jonagold, two highly susceptible cultivars, showed that some organic materials are more effective than others (see Figure 2).
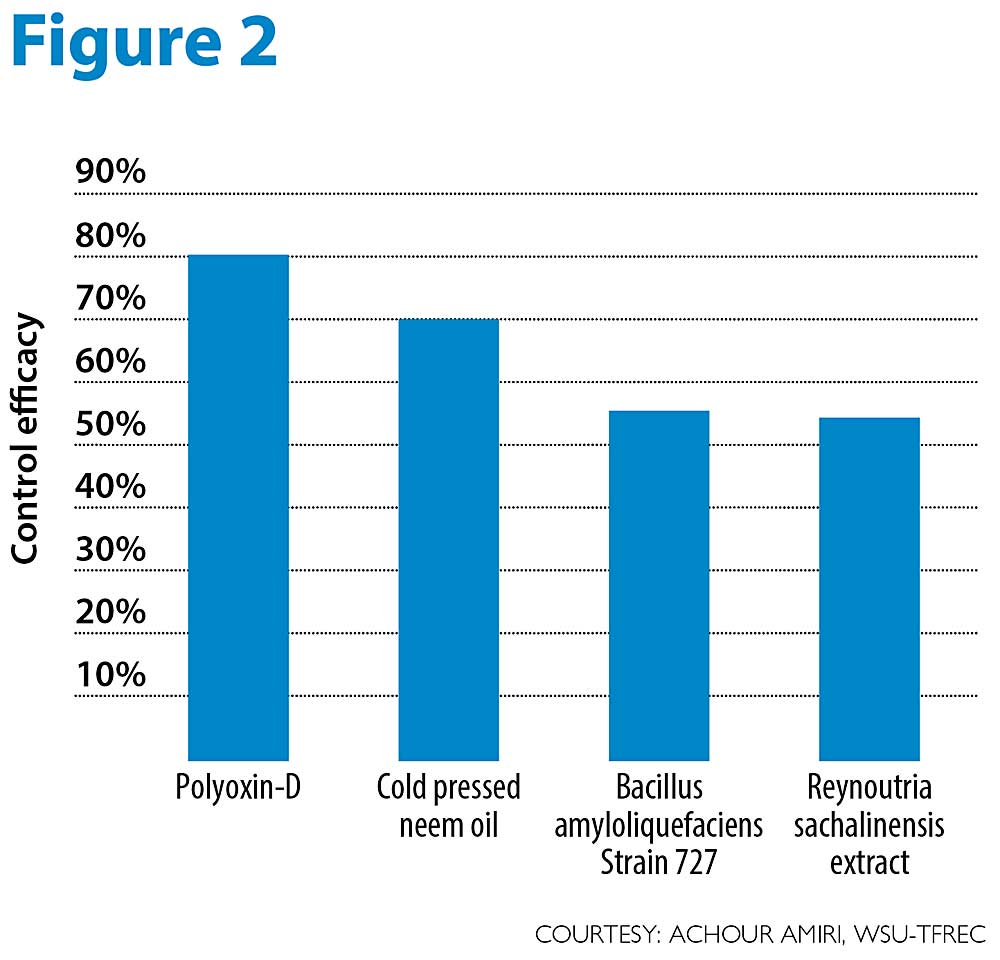
Polyoxin D zinc salt, registered as OSO, applied alone or in combination with other organic materials was effective in controlling apple mildew. Neem oil (Rango) provided up to 70 percent control, whereas the biological Bacillus amyloliquefaciens (strain 727, Stargus) and the extract of Reynoutria sachalinensis (Regalia) provided about 55 percent control under high disease pressure on highly susceptible cultivars. A rotation of these different materials with sulfur at the right timing should minimize apple mildew infections in organic orchards. We recommend using OSO and neem oil in rotation with other materials under high disease mildew pressure, while the other materials are expected to provide a sufficient control level under moderate mildew pressure or on less susceptible cultivars. •
—by Lederson Gañán-Betancur, Tobin Peever and Achour Amiri
Lederson Gañán-Betancur is a graduate research assistant at Washington State University. Tobin Peever is a former professor of plant pathology at WSU, Pullman. Achour Amiri is an assistant professor of plant pathology at the WSU Tree Fruit Research and Extension Center, Wenatchee.






Leave A Comment