One important lesson that we learned during the COVID-19 pandemic is the value of swift detection of the virus (severe acute respiratory syndrome coronavirus 2 or SARS-CoV-2) that causes the coronavirus disease. Scientists were able to generate the genome sequence of SARS-CoV-2 using cutting-edge techniques to enable rapid development of molecular testing, based on polymerase chain reaction, or PCR, for reliable detection of the virus and implement prompt and appropriate measures to prevent disease outbreaks. New frontiers in detection methods are helping to track legions of viruses circulating across communities and continents and contain the seemingly endless onslaught of viral disease outbreaks from turning into global pandemics causing death and suffering.
While much of the world is focused on containing the spread of human viral diseases, such as coronavirus, Ebola and Zika, the onslaught of viruses also continues to affect sustainability of important crops on which we depend for food and income. One such example is grapevine, the fruit crop most widely planted globally to produce wine, grape juice, fresh grapes or dried raisins. Currently, about 85 viruses have been reported from grapevines around the world, a number that far exceeds viruses reported from any other agriculturally important fruit crop. Although this high number of viruses is daunting, only a few of them are important due to their wide geographical distribution and the damaging effects they cause to vine health and fruit yield and quality.
https://www.medical-exclusive.com/generisches-stromectol-online-kaufen/In Washington state, annual surveys of wine grape vineyards during the past 15 years revealed the occurrence of 15 different viruses and their genetic variants. Among them, grapevine leafroll disease is widespread, followed by grapevine red blotch and grapevine fanleaf degeneration/decline diseases. These viral diseases are considered economically detrimental, since they are known to cause a wide range of problems that include poor vine health and vineyard longevity, reduced grape yield, delayed fruit maturity and poor quality of grapes that results in low-quality wines.
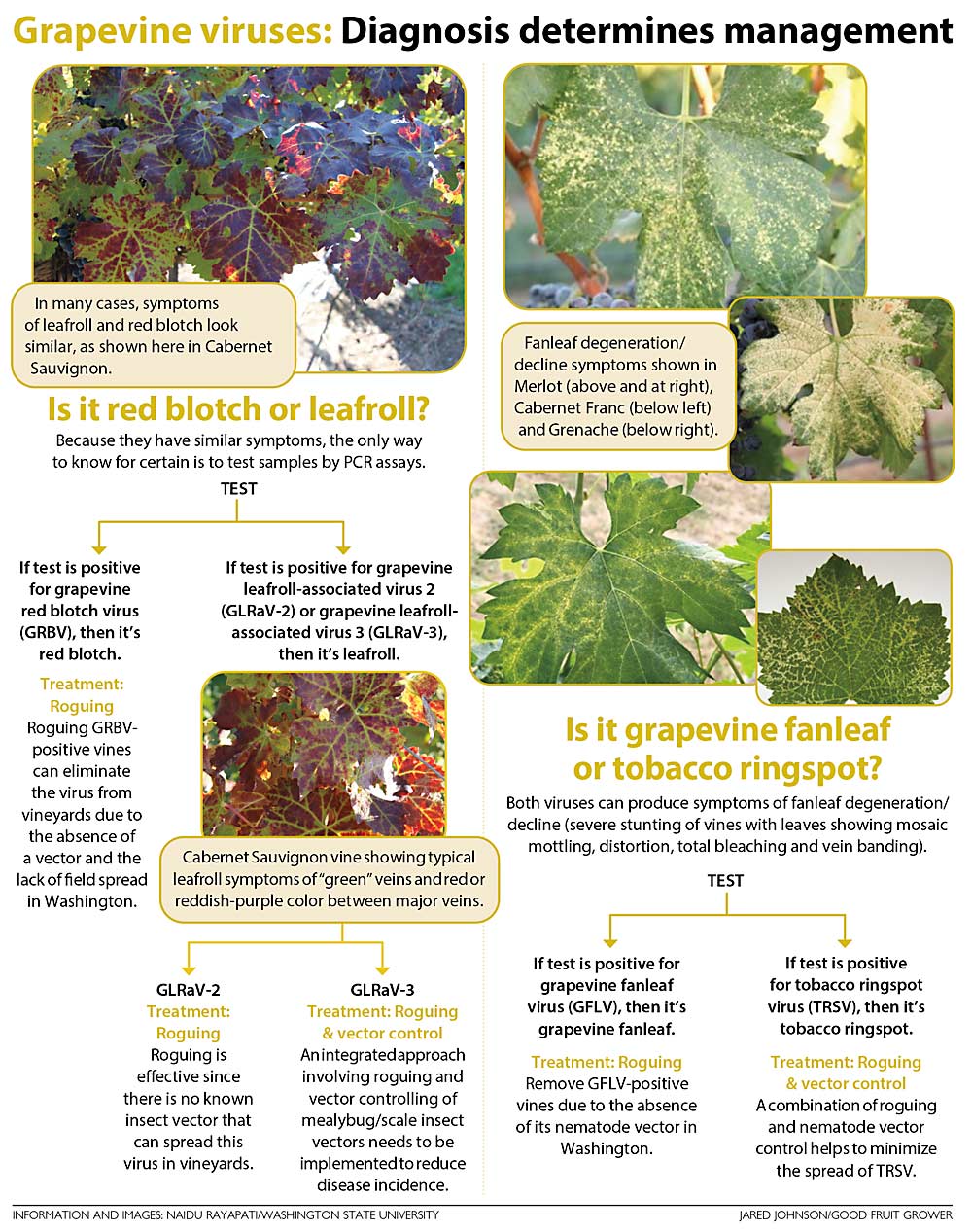
The potential significance of other viruses documented to date in vineyards is currently unknown because they occur as co-infections with other viruses or cause asymptomatic infections when present alone. Leafroll is a complex viral disease, and six distinct viruses, designated as grapevine leafroll-associated viruses (GLRaVs), were documented in grapevines. Among them, GLRaV-1, -2, -3 and -4 were documented to date in Washington vineyards, with GLRaV-3 found to be insidious and more widespread than GLRaV-1, -2 and -4. Red blotch disease is caused by grapevine red blotch virus (GRBV). A comprehensive survey indicated that GLRaV-3 is more common than GRBV in Washington vineyards, independent of cultivars and geographic region. Tobacco ringspot virus (TRSV) and grapevine fanleaf virus (GFLV) were detected in vineyards showing fanleaf symptoms. However, these two viruses were found sporadically in Washington vineyards.
Field studies have shown that GLRaV-3 can be spread within and between vineyards by grape mealybugs and soft scale insects. In contrast, vine-to-vine spread of GRBV was not observed due to the absence of a vector in Washington vineyards. Consequently, roguing, or removal of infected vines followed by replanting with virus-tested cuttings, can be used as a low-cost strategy to manage red blotch in vineyards. Roguing to control GLRaV-3 was found to be beneficial in some vineyards, while this approach was found to be less effective in other vineyards due to field spread of the virus. In general, a combination of roguing followed by replanting with virus-tested vines and vector control tactics are recommended for the management of leafroll disease.
Field studies have shown that the dagger nematode (Xiphinema rivesi) present in vineyard soils can spread TRSV from infected to healthy vines. In contrast, field spread of GFLV was not observed due to the absence of its nematode vector, X. index. Thus, a two-pronged approach, consisting of planting virus-tested cuttings and nematode control measures, is necessary for the management of TRSV. Conversely, removing infected vines and replanting with virus-tested cuttings would help prevent the spread of GFLV in vineyards due to the absence of its nematode vector.
Jared Johnson/Good Fruit Grower)
Managing viral diseases in vineyards is a top priority for Washington’s grape and wine industry. Effective management of viral diseases in vineyards relies on accurate identification of viruses infecting grapevines. Therefore, regular monitoring of vineyards and testing of symptomatic vines are two critical features of a successful endeavor to reduce virus incidence and spread and to implement disease management practices.
Although field scouting for symptoms is used to survey for viral diseases in vineyards, symptom-based identification of GLRaV-3 and GRBV for the field diagnosis of leafroll and red blotch diseases, respectively, is unreliable due to overlapping or similar symptoms in red-fruited cultivars. It is also important to emphasize that both diseases produce either mild or no apparent symptoms in white-fruited cultivars, making visual diagnosis challenging. The lack of conspicuous symptoms in white-fruited cultivars gives a false impression that they are resistant or immune to leafroll and red blotch diseases. It should be remembered that white-fruited wine grape cultivars are as susceptible to both diseases as red-fruited cultivars, irrespective of their differences in symptom expression.
Thus, reliable detection of GLRaV-3 and GRBV in both red- and white-fruited cultivars is essential to differentiate symptoms between leafroll and red blotch for implementing appropriate measures tailored to control these two distinct viral diseases in vineyards. Since red-fruited cultivars express red leaf symptoms due to viral infection as well as other biotic (crown gall infection) and abiotic (mechanical damage, cold damage and insect feeding damage) stress factors, the use of diagnostic assays will help distinguish leafroll disease and red blotch disease from “symptoms” induced by biotic and abiotic stress factors. The diagnosis benefits growers by offering appropriate guidelines to control these serious diseases. Likewise, symptoms of fanleaf degeneration/decline can be caused by GFLV, TRSV and several nematode-transmitted viruses. Just as for leafroll and red blotch, symptom-based diagnosis is less than optimal for the identification of a specific virus in symptomatic vines, and accurately identifying the virus associated with fanleaf symptoms is critical to deploying targeted control strategies.
Due to practical challenges associated with symptom-based diagnosis of leafroll, red blotch and fanleaf diseases, accurate detection of viruses present in symptomatic vines is fundamental in managing these viral diseases in vineyards. The grape virology program at Washington State University’s Irrigated Agriculture Research and Extension Center in Prosser has established robust sampling strategies and cost-effective molecular diagnostic methods for high-throughput and reliable detection of viruses in grapevines, benefiting growers, nurseries and other end users. We are also using the latest genomic analysis techniques for tracking viral genetic variants in vineyards and incorporating this knowledge for improved detection and management decision-making.
Maintaining healthy vineyards to produce premium grapes is our goal. In partnership with growers, nurseries, regulatory agencies and wine industry stakeholders, implementing science-based, best management practices is vital for mitigating negative impacts of viral diseases in vineyards. With changing viticultural practices, planting vineyards with grafted vines, adaptation to climate resiliency, deploying an array of tactics, such as using virus-tested planting stock, phytosanitary measures, roguing and chemical control of vectors, conferring an additive effect is critical for managing viral diseases and staying ahead of emerging viral disease threats to foster sustainability of Washington’s young wine industry.
—by Naidu Rayapati
Naidu Rayapati is a grape virologist and director of Washington State University’s Irrigated Agriculture Research and Extension Center in Prosser. He can be reached for questions and advice via email at naidu.Rayapati@wsu.edu or call 509-786-9215.






Leave A Comment