With antibiotic-resistant bacterial diseases becoming more difficult to control, scientists are now looking to naturally occurring bacterial killers already in the soil. Called bacteriophages, or simply phages, these assassins are viruses that have evolved over millennia to very specifically and efficiently target and destroy individual bacteria, including destructive antibiotic-resistant pathogens becoming increasingly common in orchards and vineyards.
Already, a few phage cocktails, or collections of several different phages, have made their way to the market. They include phages to fight the Xylella fastidiosa bacterium behind Pierce’s disease in grapes, Erwinia amylovora that causes fire blight in apples, and Pseudomonas syringae that leads to bacterial canker in cherries.
As an example, the phage cocktail marketed under the name XylPhi-PD by A&P Inphatec LLC of Palo Alto, California, is now approved by the Environmental Protection Agency as a treatment for Pierce’s disease and listed by the Organic Materials Review Institute for use in organic production. The company reported results from a three-year commercial field trial in a December 2021 press release: 55 percent reduction of detectable X. fastidiosa, 80–100 percent reduction in new infections, and 17 percent increase in fruit yield.
Despite the availability and effectiveness of phage cocktails seen so far, growers may want to wait a bit before relying on them to control bacterial diseases, according to Michigan State University professor George Sundin, who has been conducting research on fire blight and bacterial canker phages for several years.
“This is an area that we want growers to be aware of, and it’s something that we’re excited about and we’re working on, but it’s not quite ready for the orchard yet,” he said.
Pierce’s disease
With phages, everything old is new again. They were discovered about a hundred years ago and used to battle bacterial infections in people. But phages fell out of favor by the middle of the 20th century for several reasons, notably the discovery and rise of antibiotics. In recent years, phages again began making news as last-resort saviors for people with raging antibiotic-resistant bacterial infections, and before long, interest turned to their application for bacterial diseases in plants, said Carlos F. Gonzalez, associate director of the Texas A&M University Center for Phage Technology.
Gonzalez, a professor of plant pathology and microbiology, was already helping to generate one-off phage treatments for severe infections in people, when he began investigating them to fight Pierce’s disease.
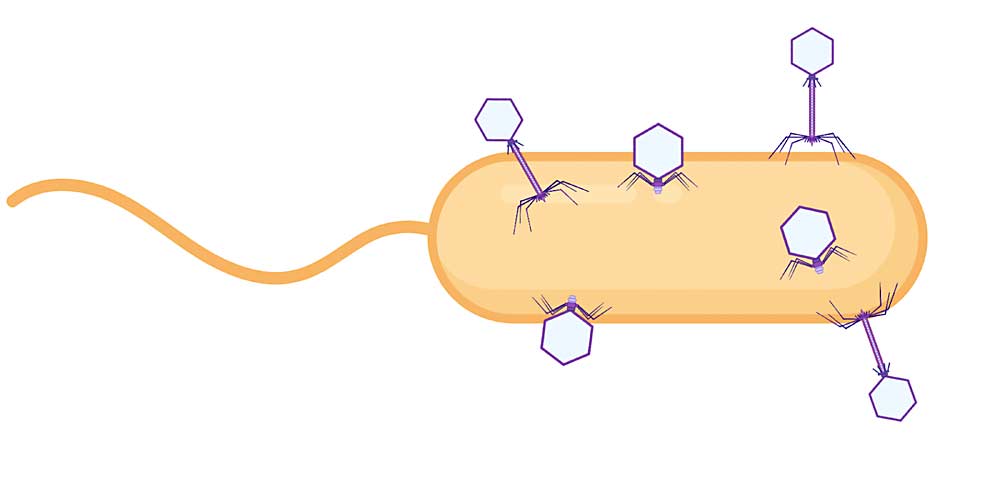
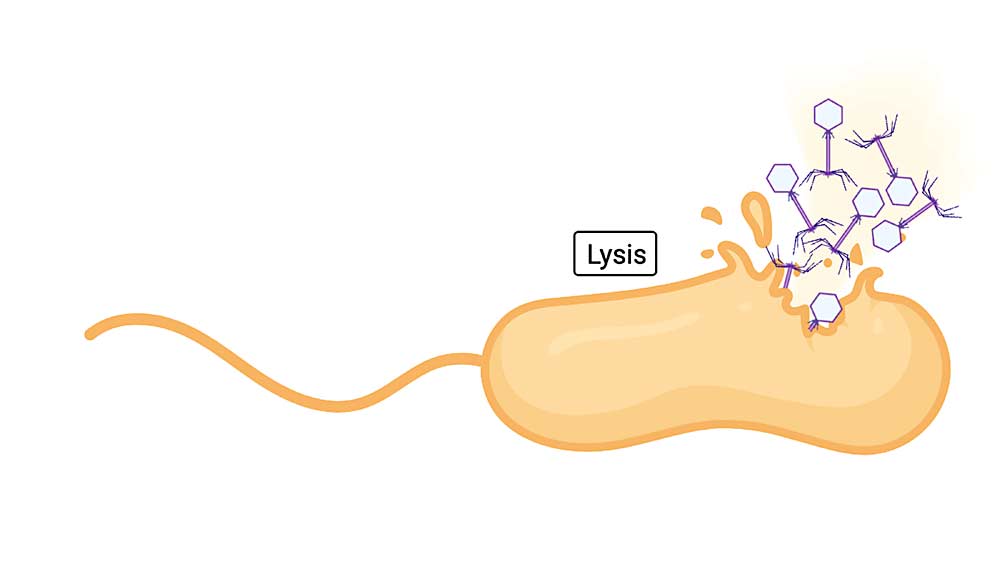
“Our laboratory became the first to isolate and characterize virulent phages for Xylella,” he said. Virulent phages work by finding the target bacterium and injecting its own DNA. This hijacks the bacterial cell’s machinery so it makes new phages. Eventually the cell bursts, releasing the new phages to go out and repeat the process.
By making cocktails that contain several phages, each homing in on different aspects of the target bacterium, kill rates are higher and the bacterium has little chance of developing resistance.
Since his lab’s achievement, research on these Xylella phages has continued, most recently with A&P Inphatec’s work to take it from lab to commercialization. Currently, XylPhi-PD is applied via injection into the stem, and A&P Inphatec is continuing to optimize the process, Gonzalez said.
Bacterial canker and fire blight
Phages are particularly attractive biocontrols, because they are so specific, Sundin said.
“The phage that attacks Pseudomonas for bacterial canker does not attack Erwinia for fire blight,” he said. “And since they don’t have a wide host range, they don’t impact beneficials that also actually may be keeping pathogen levels down.”
Besides their specificity, they offer possible alternatives for growers who need new ways to battle bacterial diseases.
“For fire blight, we use antibiotics, and so we’re always concerned about antibiotic resistance and also the possibility that the EPA might come down and say we can’t use them anymore,” he said. “And for bacterial canker, we really don’t have great methods for control. We can use copper, but there is some resistance, and also sweet cherry trees are sensitive to copper phytotoxicity.”

Sundin has been running phage trials for the past six years, some in collaboration with pathologists Sara Villani at North Carolina State University and Kerik Cox at Cornell University. Trials have included both commercially available phage cocktails and new cocktails they have developed themselves.
One of the biggest success stories so far is a phage cocktail Sundin’s group specifically targeted to the hundreds of slightly different strains of fire blight bacterium found in northern Michigan apple orchards. In their lab tests, the commercially available phage cocktails killed about 60 percent of the strains, but their cocktail comprising phages they collected from local soil killed 98 percent, he said. They hope to test it in the field this season.
One of the challenges with phages, and one Sundin and colleagues are concentrating on now, is phage delivery.
“When the pathogens are present on a flower, they’re present at really high concentrations and they’re all stacked up on each other,” he said. That doesn’t pose a problem for antibiotic sprays, because the water-antibiotic mix diffuses throughout the flower. Because phages are physical particles that do not dissolve in water, however, a spray may not move them through the bacterial cells as well, which can reduce their efficacy.
Nonetheless, he remains optimistic about phages because their field tests show most of the tested cocktails provide 50 percent control, “which is what we get with other biological agents, such as Serenade, the popular biological for fire blight,” he said.
Environmental conditions, such as ultraviolet radiation or aridity, may also affect phages in the field, so these considerations all require further thought and research.
“The bottom line is that we’re excited about phages, but as we’ve worked with them, we have come to understand that there are challenges in order to optimize them,” Sundin said. “Phage development is taking longer than we thought, but hopefully we’re addressing the difficulties and, once we figure these things out, we’ll have a much better working material for growers.”
—by Leslie Mertz






Leave A Comment